|
Listen to this article
|

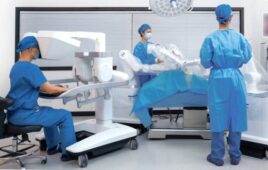
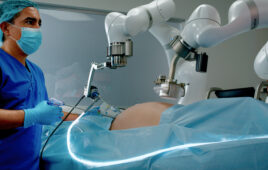
The Misso robot will help surgeons with personalized pre-planning and precise cutting for joint replacement. | Source: Meril
Meril Life Sciences Pvt. Ltd. this week unveiled its Misso surgical robot for simplifying orthopedic procedures.
The India-based company announced on LinkedIn that it is developing surgical robot for total knee procedures. It said it designed Misso to help surgeons with personalized pre-planning and precise cutting for joint replacements.
Misso has an optical tracking sensor, a six-axis articulated robotic arm, and “superior” safety systems, claimed Meril. The surgical robot can help physicians perform real-time gap checks and modify a surgical plan intraoperatively, it explained.
Various cutting options are available on the Misso, including full and partial cutting based on surgeon preference. Cutting guides are not required, and the system can perform a tibial cut and a fully finished femur with all the cuts, including peg holes, according to the company.
Misso includes safety features
Meril designed Misso with bone-movement monitoring to ensure safety during procedures. If bone movement is observed, the resection process stops.
The resection will continue with automatic recovery of the bone position. If the robotic system’s tools contact the patient or other object, the collaborative robot arm will immediately stop moving.
Misso uses optical tracking-based navigation and robotic assistance to ensure accurate results in shaping and aligning artificial knee joint components. The company aims to improve post-operative outcomes, reduce complications, and promote faster patient recovery.
Meril joins competitive surgical robot market
Founded in 2006, Meril said it is dedicated to the innovation, design, and development of novel, clinically relevant devices. Its product portfolio includes vascular intervention devices, orthopedic implants, robotics, endo-surgery, ENT products, and in-vitro diagnostics.
The company has joined the growing surgical robotics market, where it will compete with orthopedic surgical robot developers such as Stryker and Zimmer Biomet. Stryker launched its its Mako surgical robot in 2017 and introduced a joint-replacement offering in February 2024.
Also in February, the U.S. Food and Drug Administration (FDA) cleared Zimmer Biomet’s Rosa surgical robotic platform for shoulder and hip replacement procedures. The FDA had cleared it for partial knee replacement for partial knee replacement in April 2021.
Editor’s note: This article is syndicated from The Robot Report sibling site MassDevice.



Tell Us What You Think!